1) Compare the properties of
electrons, protons and neutrons.
electrons, protons and neutrons.
ANSWER:-Electrons- Electrons are
Negatively charged particles Protons-Protons are
Positively charged particles. Neutron-Neutrons do not
carry any charge and are neutral
Negatively charged particles Protons-Protons are
Positively charged particles. Neutron-Neutrons do not
carry any charge and are neutral
2) What are the limitations of
J.J. Thomson’s model of the
atom?
J.J. Thomson’s model of the
atom?
ANSWER:-The limitations of J.J. Thomson’s
model of the atom are: → It could not explain the result of scattering experiment performed
by Rutherford.
model of the atom are: → It could not explain the result of scattering experiment performed
by Rutherford.
→ It did not have any experiment support.
3) What are the limitations of Rutherford’s model of the atom?
ANSWER;-The limitations of Rutherford’s model of the atom are→ It failed to explain the stability of an atom.
→ It doesn’t explain the spectrum of hydrogen and other
atoms.
atoms.
4) Describe Bohr’s model of the atom.
ANSWER:-→ The atom consists of a small positively charged nucleus at its
center.
center.
→ The whole mass of the
atom is concentrated at the nucleus and the volume of the nucleus is much
smaller than the volume of the atom.
atom is concentrated at the nucleus and the volume of the nucleus is much
smaller than the volume of the atom.
→ All the protons and
neutrons of the atom are contained
in the nucleus.
neutrons of the atom are contained
in the nucleus.
→ Only certain orbits
known as discrete orbits of
electrons are allowed inside the atom.
known as discrete orbits of
electrons are allowed inside the atom.
→ while revolving in these discrete orbits electrons do not radiate energy. These orbits or cells
are represented by the letters K, L, M, N etc.
are represented by the letters K, L, M, N etc.
6) Summarize the rules for writing of distribution of electrons in various shells for the
first eighteen elements.
first eighteen elements.
ANSWER:-→ If n gives the number of orbit or energy level, then 2n 2 gives the maximum number of electrons
possible in a given orbit or energy level.
Thus, First orbit or K-shell will have 2 electrons, Second orbit or L-shell will have 8 electrons, Third orbit or M-shell will have 18 electrons.
possible in a given orbit or energy level.
Thus, First orbit or K-shell will have 2 electrons, Second orbit or L-shell will have 8 electrons, Third orbit or M-shell will have 18 electrons.
→ If it is the outermost
orbit, then it should have not more than
8 electrons.
orbit, then it should have not more than
8 electrons.
→ There should be
step-wise filling of electrons in
different orbits, i.e., electrons
are not accompanied in a given
orbit if the earlier orbits or
shells are incompletely filled.
step-wise filling of electrons in
different orbits, i.e., electrons
are not accompanied in a given
orbit if the earlier orbits or
shells are incompletely filled.
7) Define valency by taking examples of silicon and oxygen.
ANSWER:-The valency of an element
is determined by the number of valence
electrons present in the atom of
that element.→ Valency of Silicon: It has electronic configuration: 2, 8, 4 Thus, the valency of silicon is 4 as these electrons can
be shared with others to complete octet.
is determined by the number of valence
electrons present in the atom of
that element.→ Valency of Silicon: It has electronic configuration: 2, 8, 4 Thus, the valency of silicon is 4 as these electrons can
be shared with others to complete octet.
→ Valency of Oxygen: It
has electronic configuration: 2, 6
has electronic configuration: 2, 6
Thus, the valency of oxygen is 2
as it will gain 2 electrons to complete its octet.
as it will gain 2 electrons to complete its octet.
8) Explain with examples (i) Atomic number, (ii) Mass number, (iii) Isotopes and (iv) Isobars. Give any two uses of
isotopes.
isotopes.
ANSWER 🙁 i) Atomic number: The atomic number of an element is
the total number of protons present in the atom of
that element. For example, nitrogen has 7 protons in its atom. Thus,
the atomic number of nitrogen is 7.
the total number of protons present in the atom of
that element. For example, nitrogen has 7 protons in its atom. Thus,
the atomic number of nitrogen is 7.
(ii) Mass number: The mass number
of an element is the sum of the number of
protons and neutrons present in
the atom of that element. For example, the atom of boron has
5 protons and 6 neutrons.
of an element is the sum of the number of
protons and neutrons present in
the atom of that element. For example, the atom of boron has
5 protons and 6 neutrons.
So, the mass number of boron is
5 + 6 = 11.
5 + 6 = 11.
(iii) Isotopes: These are atoms of
the same element having the same atomic number,
but different mass numbers.
the same element having the same atomic number,
but different mass numbers.
(iv) Isobars: These are atoms having
the same mass number, but different atomic
numbers i.e., isobars are atoms
of different elements having the same mass number.
the same mass number, but different atomic
numbers i.e., isobars are atoms
of different elements having the same mass number.
Two uses of isotopes:
→ One isotope of uranium
is used as a fuel in nuclear
reactors.
is used as a fuel in nuclear
reactors.
→ One isotope of cobalt is used in the treatment of cancer.
9) Na + has completely filled K and L shells. Explain.
ANSWER:-The atomic number of sodium is 11. So, neutral sodium atom
has 11 electrons and its electronic configuration
is 2, 8, 1. But Na + has 10
electrons. Out of 10, K-shell
contains 2 and L-shell 8 electrons
respectively. Thus, Na + has completely filled K and L shells.
has 11 electrons and its electronic configuration
is 2, 8, 1. But Na + has 10
electrons. Out of 10, K-shell
contains 2 and L-shell 8 electrons
respectively. Thus, Na + has completely filled K and L shells.
10). If bromine atom is available in the form of, say, two
isotopes 79 / 35Br (49.7%) and 81 / 35Br (50.3%), calculate the average atomic
mass of bromine atom.
isotopes 79 / 35Br (49.7%) and 81 / 35Br (50.3%), calculate the average atomic
mass of bromine atom.
ANSWER:-
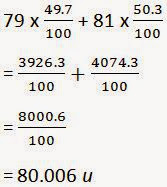
11) The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 16 / 8 X and 18 / 8
X in the sample?
X in the sample?
ANSWER:-Let the percentage of isotope
18 / 8 X be y%. Thus, the percentage of isotope
16 / 8 X will be (100 – y) %.
18 / 8 X be y%. Thus, the percentage of isotope
16 / 8 X will be (100 – y) %.
Therefore,
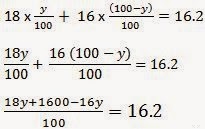
18y + 1600 – 16y = 1620
2y + 1600 = 1620
2y = 1620 – 1600
y= 10
Therefore, the percentage
ofisotope 18 / 8 X is 10%.
ofisotope 18 / 8 X is 10%.
And, the percentage of isotope
16 / 8 X is (100 – 10) % = 90%.
12) If Z = 3, what would be the
valency of the element? Also, name the element.
valency of the element? Also, name the element.
ANSWER:-By Z = 3, we mean that the atomic number of the element is
3. Its electronic configuration is 2, 1.
Hence, the valency of the
element is 1 (since the outermost shell has
only one electron).
3. Its electronic configuration is 2, 1.
Hence, the valency of the
element is 1 (since the outermost shell has
only one electron).
Therefore, the element with Z = 3
is lithium.
is lithium.
13) Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons = 6. 8
Give the mass numbers of X and Y. What is the relation between the two species?
ANSWER:-Mass number of X = Number
of protons + Number of neutrons
of protons + Number of neutrons
= 6 + 6
= 12
Mass number of Y = Number of
protons + Number of neutrons
protons + Number of neutrons
= 6 + 8
= 14
These two atomic species X and
Y have the same atomic number, but different
mass numbers. Hence, they are isotopes
Y have the same atomic number, but different
mass numbers. Hence, they are isotopes
14) For the following
statements, write T for ‘True’ and F for ‘False’.
statements, write T for ‘True’ and F for ‘False’.
(a) J.J. Thomson proposed
that the nucleus of an atom
contains only nucleons.
that the nucleus of an atom
contains only nucleons.
► False
(b) A neutron is formed by an
electron and a proton combining together. Therefore, it is neutral.
electron and a proton combining together. Therefore, it is neutral.
► False
(c)
The mass of an electron is about 1 / 2000times
that of proton.
The mass of an electron is about 1 / 2000times
that of proton.
► True
(d) An isotope of iodine is
used for making tincture iodine, which is used as a medicine.
used for making tincture iodine, which is used as a medicine.
► False
15) Rutherford’s alpha-particle scattering
experiment was responsible
for the discovery of
experiment was responsible
for the discovery of
(a) Atomic nucleus
(b) Electron
(c) Proton
(d) Neutron
ANSWER:-a) Atomic nucleus
16) Isotopes of an element have
(a) The same physical
properties
properties
(b) Different chemical
properties
properties
(c) Different number of
neutrons
neutrons
(d) Different atomic numbers
ANSWER:-different number of neutrons
17) Number of valence electrons
in Cl – ion are:
in Cl – ion are:
(a) 16
(b) 8
(c) 17
(d) 18
ANSWER:-b) 8
18) Which one of the following
is a correct electronic configuration of sodium?
is a correct electronic configuration of sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1
ANSWER:-d) 2, 8, 1
19) Complete the following
table. Atomic num. Mass num. No of
Neutrons
table. Atomic num. Mass num. No of
Neutrons
9. 19. 10
16. 32. 16
12. 24. 12
1. 2. 1
1. 1. 0