
(Page-20-21)
1) Ethane,
with the molecular formula C2H6 has
with the molecular formula C2H6 has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
ANSWER:-Ethane has 7 covalent bonds.
2) Butanone is a four-carbon compound with the
functional group
functional group
(a) Carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
ANSWER:-The
functional group of butanone is ketone.
functional group of butanone is ketone.
3) While cooking, if the bottom of the vessel is
getting blackened on the outside, it means that
getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
ANSWER:-While cooking, if the bottom of the vessel is
getting blackened on the outside, then it means that the fuel is not burning
completely.
getting blackened on the outside, then it means that the fuel is not burning
completely.
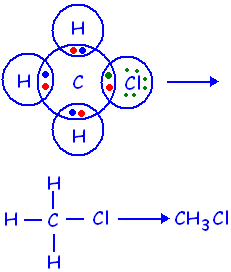
4) Explain the nature of the covalent bond using the
bond formation in CH3Cl.
bond formation in CH3Cl.
ANSWER:-Carbon neither gains nor looses electrons as
it has a valency of four .it completes its octet by sharing its four
electrons with other carbon atoms or with atoms of other elements. The bonds
that are formed by sharing electrons are known as covalent bonds. In covalent
bonding, both the atoms share the valence electrons, i.e., the shared electrons
belong to the valence shells of both the atoms. Here, carbon requires 4
electrons to complete its octet, while each hydrogen atom requires one electron
to complete its duplet. Also, chlorine requires an electron to complete the octet.
Therefore, all of these share the electrons and as a result, carbon forms 3
bonds with hydrogen and one with chlorine.
it has a valency of four .it completes its octet by sharing its four
electrons with other carbon atoms or with atoms of other elements. The bonds
that are formed by sharing electrons are known as covalent bonds. In covalent
bonding, both the atoms share the valence electrons, i.e., the shared electrons
belong to the valence shells of both the atoms. Here, carbon requires 4
electrons to complete its octet, while each hydrogen atom requires one electron
to complete its duplet. Also, chlorine requires an electron to complete the octet.
Therefore, all of these share the electrons and as a result, carbon forms 3
bonds with hydrogen and one with chlorine.
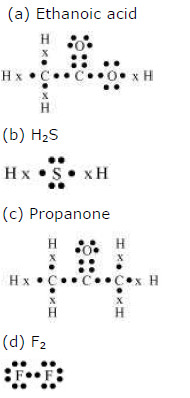
5)Draw the electron dot structures for
(a) ethanoic acid.
(b) H2S.
(c) propanone.
(d) F2.
ANSWER:-
6) What is a homologous series? Explain with an example.
ANSWER:-A homologous series is a series of carbon
compounds that have different numbers of carbon atoms but contain the same
functional group.
compounds that have different numbers of carbon atoms but contain the same
functional group.
Example:-The general formula
of this series is CnH2n+2.
Methane CH4
Ethane CH3CH3
Propane CH3CH2CH3
Butane CH3CH2CH2CH3
It can be noticed that there is a difference of −CH2 unit between each successive compound.
7) How can ethanol and ethanoic acid be
differentiated on the basis of their physical and chemical properties?
differentiated on the basis of their physical and chemical properties?
ANSWER:-Ethanol is a liquid at room temperature with a
pleasant
odour and ethanoic acid has vinegar-like smell.
pleasant
odour and ethanoic acid has vinegar-like smell.
Ethanoic acid reacts with metal carbonates and metal
hydrogen carbonates to form salt, water, and carbon dioxide gas while ethanol
does not react with them.
hydrogen carbonates to form salt, water, and carbon dioxide gas while ethanol
does not react with them.
8) Why does micillie formation takes place when soap
is added to water? Will a micillie be formed in other solvents such as ethanol also?
is added to water? Will a micillie be formed in other solvents such as ethanol also?
ANSWER:- Due to the molecules that have a unique
orientation that keeps the hydrocarbon portion out of the water micillie
formation takes place.
orientation that keeps the hydrocarbon portion out of the water micillie
formation takes place.

9) Why are carbon and its compounds used as fuels
for most applications?
for most applications?
ANSWER:-Carbon and its compounds are used as fuels for
most applications as because most of the carbon compounds give a lot of heat
and light when burnt in air. Saturated hydrocarbons burn with a clean flame and
no smoke is produced.
most applications as because most of the carbon compounds give a lot of heat
and light when burnt in air. Saturated hydrocarbons burn with a clean flame and
no smoke is produced.
10) Explain the formation of scum when hard water is
treated with soap.
treated with soap.
ANSWER:-Soap does not work properly when the water is
hard.
A soap is a sodium or potassium salt of long chain fatty acids. Hard water
contains salts of calcium and magnesium. When soap is added to hard
water, calcium and magnesium ions present in water displace sodium
or potassium ions from the soap molecules forming an insoluble substance called
scum.
hard.
A soap is a sodium or potassium salt of long chain fatty acids. Hard water
contains salts of calcium and magnesium. When soap is added to hard
water, calcium and magnesium ions present in water displace sodium
or potassium ions from the soap molecules forming an insoluble substance called
scum.
11) What change will you observe if you test soap
with litmus paper (red and blue)?
with litmus paper (red and blue)?
ANSWER:-It will
turn red litmus blue. However, the colour of blue litmus will
remain blue.
turn red litmus blue. However, the colour of blue litmus will
remain blue.
12) What is hydrogenation? What is its industrial
application?
application?
ANSWER:-Process
of addition of hydrogen is known as hydrogenation. Hydrogenation is
widely applied to the processing of vegetable oils and fats.
of addition of hydrogen is known as hydrogenation. Hydrogenation is
widely applied to the processing of vegetable oils and fats.

13) Which of the following hydrocarbons undergo addition reactions: C2H6, C3H8,
C3H6, C2H2 and CH4.
C3H6, C2H2 and CH4.
ANSWER:-C3H6 and C2H2 undergo addition reactions.
14) Give a test that can be used to differentiate
chemically between butter and cooking oil.
chemically between butter and cooking oil.
ANSWER:-Bromine water test can be used to
differentiate chemically between butter and cooking oil. Add bromine water to a
little of cooking oil and butter taken in separate test tubes.
differentiate chemically between butter and cooking oil. Add bromine water to a
little of cooking oil and butter taken in separate test tubes.
a. Decolourising of bromine water by cooking oil (unsaturated
compound)
compound)
b. Butter (saturated compound) does not decolourise
bromine water
bromine water
15) Explain the mechanism of the cleaning action of
soaps .
soaps .
ANSWER:- A soap molecule has two parts, a
head and a tail i .e. the long chain organic part and the functional
group –COO Na .The organic part
is water insoluble but is soluble in organic solvents or in oil or grease . The
ionic part is soluble in water, as water is a polar solvent. When soap is added
to water in which dirty clothes are soaked, the two parts
of the soap molecule dissolve in two different mediums. The organic tail
dissolves in the dirt, grime or grease and the ionic head dissolves
in water. When the clothes are rinsed or agitated, the dirt gets pulled out of
the clothes, by the soap molecule. In this way soap does its cleaning work on
dirty and grimy clothes or hands.
head and a tail i .e. the long chain organic part and the functional
group –COO Na .The organic part
is water insoluble but is soluble in organic solvents or in oil or grease . The
ionic part is soluble in water, as water is a polar solvent. When soap is added
to water in which dirty clothes are soaked, the two parts
of the soap molecule dissolve in two different mediums. The organic tail
dissolves in the dirt, grime or grease and the ionic head dissolves
in water. When the clothes are rinsed or agitated, the dirt gets pulled out of
the clothes, by the soap molecule. In this way soap does its cleaning work on
dirty and grimy clothes or hands.
The soap molecules actually form a closed structure
because of mutual repulsion of the positively charged heads. This structure is
called a micelle. The micelle pulls out the dirt and grime more
efficiently .
because of mutual repulsion of the positively charged heads. This structure is
called a micelle. The micelle pulls out the dirt and grime more
efficiently .